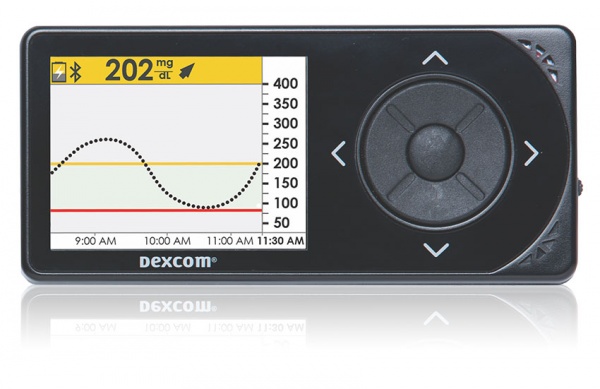
Users of the Dexcom G5 Mobile Continuous Glucose Monitoring System (Dexcom G5) can now rely on the device to make diabetes treatment decisions using two finger pricks daily, not multiple ones, following the U.S. Food and Drug Administration’s (FDA) approval of expanded use of the device.
Users must still calibrate the device at least two times a day by testing a fingertip blood sample with a blood glucose meter. Many industry experts hailed the decision as a welcome change, with some calling it a game changer and others noting that the FDA approval is simply the official blessing on what has been a common practice.
As users of the Dexcom G5, the glucose readings are generally good, however there are times that the G5 can lag significantly, particularly during times of rapid glucose changes. Given, that our recommendation is to only use G5 readings when a finger prick is not practical or if glucose levels have been steady or there has been a recent calibration.